Medical details: kwashiorkor and shock
Background
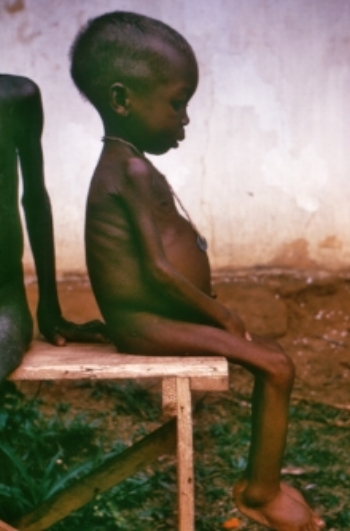
Severe protein-calorie malnutrition often results in oedema (swollen hands and feet) and ascites (swollen abdomen), when it may be termed kwashiorkor. This name means ‘displaced’ and occurs in infants who are no longer being breast-fed (often displaced by a new sibling) and who receive very little dietary protein.
Infants with kwashiorkor have very low plasma albumin levels, and it had long been assumed that their oedema was a result of this hypoalbuminaemia, causing plasma water to leak out of the capillaries. However, this view changed dramatically after a paper published in 1980 which appeared to show that this was not the case [follow link to this paper, below]. When these authors fed children who had kwashiorkor, they reported that their oedema reduced before their plasma albumin levels had risen, and so concluded that their oedema must be due to some cause other than hypoalbuminaemia. Since then there has been much work to establish possible mechanisms for the oedema, but it has remained an enigma.
The belief that kwashiorkor oedema was not caused by hypoalbuminaemia, combined with a fear of causing pulmonary oedema by using intravenous albumin, or even saline, led the WHO to advise resuscitating shocked children with this condition with small volumes of hypotonic fluid only. This is standard practice world-wide, and the mortality remains at about 50%.
Faced with these facts first hand in Mbarara, Uganda, this did not make sense to Malcolm as a physiologist. He therefore sought out Golden’s original 1980 Lancet paper for a closer look. Surprisingly, the data which led to their radical conclusion was presented as a graph just 3 x 4 cm, and without any statistical analysis [link to 1980 paper, below].
Child with kwashiorkor
Re-analysis
Malcolm scanned this graph at high resolution to obtain the primary data values, and then reworked them. He was able to show that the authors had misinterpreted their results, and that the albumin did rise prior to the loss of oedema, with a certainty of 99.999% (p<0.0001). This has been published in the International Journal of Paediatrics and Child Health [2015, link below].
Malcolm concluded that Golden had been wrong about the role of hypoalbuminaemia, and that his incorrect analysis had led to a series of papers after 1980 which were looking for other possible mechanisms for the oedema of kwashiorkor. He re-analysed these papers, and showed that they had failed to analyse their own albumin data. He extracted their data from their tables, and showed that they all showed that the oedema was linked to low plasma albumin concentrations [2015 paper, link below].
Malcolm’s 2015 paper predicted that it would be far more effective, and safe, to treat children with kwashiorkor + shock with a small dose of intravenous albumin, followed by furosemide, as we do in children with the physiologically parallel situation, with severe congenital nephrotic syndrome. He argued the need for a study to compare the existing WHO treatment (infusions with small volumes of hypotonic crystalloid) with 1 mg/kg of albumin + furosemide. The purpose of this project is to carry out that study.
Malcolm's re-analysis of Golden's data
The graph which changed practices in 1980
Initially proposed study details
Overall design After undertaking a closely-monitored Proof-of-Concept Pilot study, we aim to progress to an open block-randomised cross-over controlled trial of treating children who have kwashiorkor + shock, either by the standardised WHO fluid regimen, or by an albumin infusion, randomised in balanced blocks of eight.
Primary outcome measure To avoid using mortality at 48 hours (a typical study design), we will evaluate their short-term (4-hour) physiological responses to therapy by measuring their central to peripheral temperature gradient (C-PT). See below for details. As soon as they exit the study, they will be eligible to enrol for the alternative treatment regimen. Hence, all enrolled infants will be able to benefit from both approaches so long as they survive 4 hours (this will be shortened to 1 hours if they clinically deteriorate; see criteria below).
Secondary outcome measures will include biochemical and other clinical measures of prediction of shock, and their improvement on therapy, and morbidity and mortality rates.
Subjects Children <5 years, who the following … (1) Kwashiorkor = mid-arm circumference <115 mm or weight-for-height <3SD AND bilateral pedal oedema AND <2+ albuminuria), and (2) Shock = cold hands AND CRT ≥3 seconds AND weak, rapid pulse, and (3) don’t have pre-existing kidney or congenital heart disease.
Informed consent Parents or guardians will be given an information sheet in their local language and a full explanation by the admitting doctor or nurse. Children not enrolled will be offered standard WHO treatment.
C-PT measurements Standardly, this is measured using 2 thermistors, one in the rectum and one taped to the big toe, using the following definitions: IMPROVEMENT = fall in C-PT (narrowing of the gap) by ≥5°C, or reaching an absolute value of ≤2°C; WORSENING = gap narrows by <5°C, with an absolute value of >2°C. A deterioration sufficient to stop the study = C-PT increases of ≥2°C after first hour.
Confirmation of initial hypovolaemia will be made by measuring changes in the diameter of the inferior vena cava, using ultrasound at 0 and 4 hours.
Treatment regimens All children will receive the full recommended WHO non-fluid management regimen for acute malnutrition; only the fluid prescription will differ.
WHO fluids Hour 1; 15 ml/kg Ringer’s lactate or half-strength Darrow’s solution + 5% glucose, to repeat if there is clinical improvement. Subsequent hours; oral or ng ReSoMaL at 10 ml/kg/h to replace IV fluids, then F-75 milk formula, or continue IV fluids at 4 ml/kg/h, and cross-match whole blood to give at 10 ml/kg over 3hr.
Albumin fluids Hours 1 & 2; - infuse 5 ml/kg of 20% albumin over 2 hours, and give 2 mg/kg IV furosemide 1 hour after starting the albumin, or at once if signs of pulmonary oedema (allowed to give a second dose). After 2 hours: introduce ReSoMaL at 10 ml/kg/h, or IV 0.45% saline + 5% glucose at 4 ml/kg/h, and cross-match whole blood to give at 10 ml/kg over 3 hr.